Katayama Lab conducts education and research in the field of energy chemistry engineering.
Interdisciplinary Graduate School of Engineering Sciences (IGSES),
Kyushu University, JAPAN
Kyushu University, JAPAN
 Hydrogen Energy
Hydrogen Energy
Hydrogen is attracting attention as a clean secondary energy with a small
environmental load. When hydrogen is combusted, only water and energy are
generated but any harmful elements are not released. It is also expected
as an effective storage method for surplus energy. There are several technologies
to utilize hydrogen energy such as hyderogen engine and fuel cells. A hydrogen
engine is a way to use hydrogen like gasoline. A fuel cell is a method
to generate thermal energy and electrical energy by chemically reacting
hydrogen and oxygen. By utilizing heat and electricity, high efficiency
can be expected. There are various types of fuel cells such as solid polymer
type and solid oxide type.In our laboratory, we have conducted "Study on fundamental transport phenomena in proton conductive solid oxides" and "Study on water vapor behavior in electrolyte membranes for polymer electrolyte fuel cells". Going back further, we have actively carried out researches relating to hydrogen utilization, such as "Heat pumps using hydrogen storage alloys" and "Hydrogen isotope separation using hydrides". Recently, we are working on research related to hydrogen production.
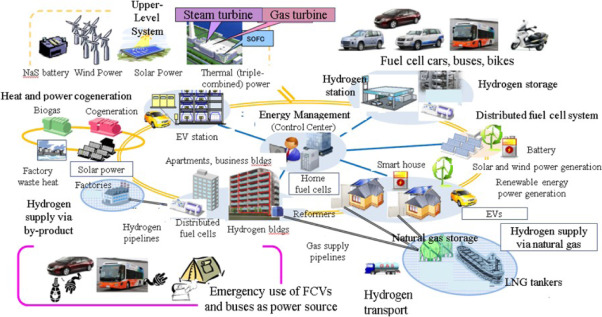
Noriko Behling et al., Economic Analysis and policy, 48 (2015) 204-221.
Related research in our Lab.
- Advancement of hydrogen production by steam reforming method
- Hydrogen producton by plasma direct decomposition method
- Hydrogen production from high temperature steam by solid oxide electrolytic cell
In this research, hydrogen by high-temperature steam electrolysis is aimed at understanding mass transfer phenomena through material characteristics and performance evaluation of solid oxide electrolysis cells (SOEC) using high-temperature heat sources toward the realization of carbon-free hydrogen production technology. Recently, we built the experimental setup and tried to acumulate experimental data at high temperature conditions. Manufacturing electrodes and gas seals that can withstand high temperatures conditions is not easy and our challenge is continued.
Contents
(since 2015/6/10)
Current viewers
Address
Katayama Laboratory
〒816-8580
6-1, Kasuga-koen, Kasuga, Fukuoka, JAPAN,
H Building, Chikushi Campus